Bayesian Sparse Graphical Models for Classification with Application to Protein Expression Data
Abstract
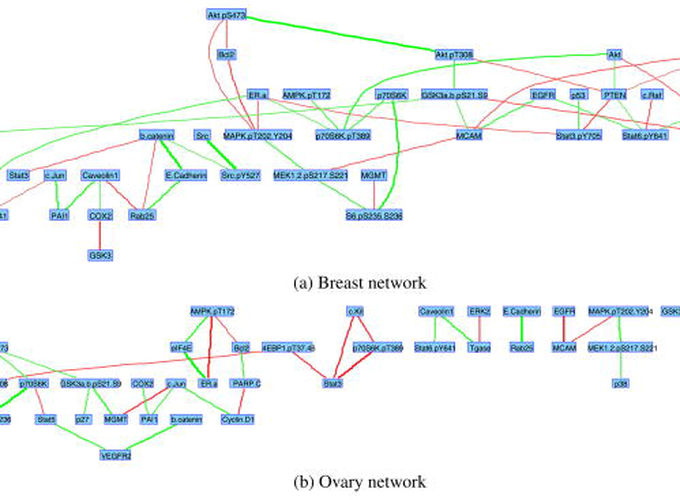
Reverse-phase protein array (RPPA) analysis is a powerful, relatively new platform that allows for high-throughput, quantitative analysis of protein networks. One of the challenges that currently limit the potential of this technology is the lack of methods that allow for accurate data modeling and identification of related networks and samples. Such models may improve the accuracy of biological sample classification based on patterns of protein network activation and provide insight into the distinct biological relationships underlying different types of cancer. Motivated by RPPA data, we propose a Bayesian sparse graphical modeling approach that uses selection priors on the conditional relationships in the presence of class information. The novelty of our Bayesian model lies in the ability to draw information from the network data as well as from the associated categorical outcome in a unified hierarchical model for classification. In addition, our method allows for intuitive integration of a priori network information directly in the model and allows for posterior inference on the network topologies both within and between classes. Applying our methodology to an RPPA data set generated from panels of human breast cancer and ovarian cancer cell lines, we demonstrate that the model is able to distinguish the different cancer cell types more accurately than several existing models and to identify differential regulation of components of a critical signaling network (the PI3K-AKT pathway) between these two types of cancer. This approach represents a powerful new tool that can be used to improve our understanding of protein networks in cancer.